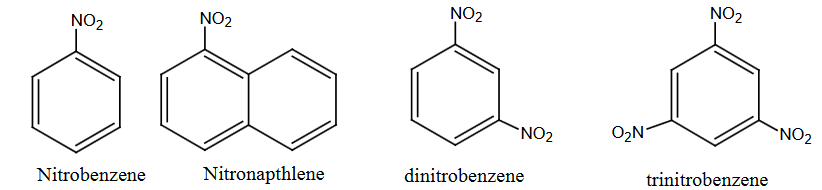
Aromatic Nitro Compounds:
When nitro groups are directly attached to the aromatic rings, such nitro compounds are aromatic nitro compounds.
Examples:
- Nitrobenzene
- Nitronaphthalene
- Dinitrobenzene
- Trinitrobenzene
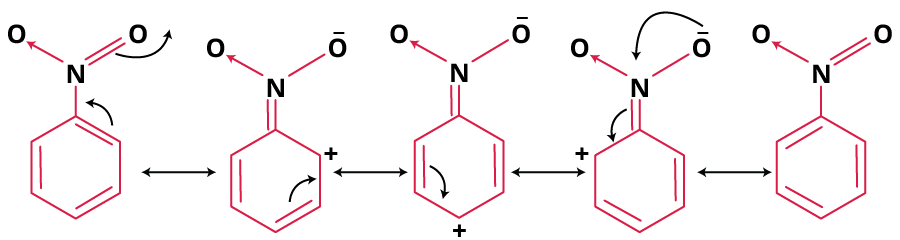
Resonance in Nitrobenzene:
Nitrobenzene exhibits resonance, resulting in several resonance structures that contribute to the stability of the molecule.

In these resonance structures, the electron density is delocalized over the aromatic ring and the nitro group, contributing to the overall stability of nitrobenzene.
Resonance in Nitrobenzene
Nitrobenzene exhibits resonance, which contributes to its stability. The resonance structures involve the delocalization of electrons from the nitro group (NO₂) across the aromatic ring. This can be illustrated as follows:
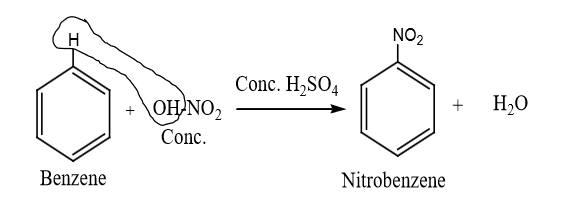
Laboratory Preparation of Nitrobenzene
Nitrobenzene can be prepared in the laboratory through the nitration of benzene using a nitrating mixture, which consists of concentrated nitric acid (HNO₃) and concentrated sulfuric acid (H₂SO₄).
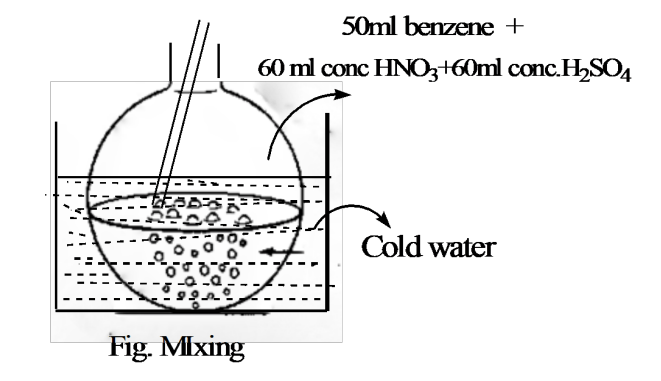
Laboratory Preparation of Nitrobenzene: Detailed Procedure
Procedure:
- Setup:
- Take 50 ml of benzene in a round-bottom (R.B.) flask.
- Addition of Nitrating Mixture:
- Prepare the nitrating mixture by mixing 60 ml of concentrated HNO₃ and 60 ml of concentrated H₂SO₄.
- Add the nitrating mixture to the R.B. flask containing benzene in portions.
- During the addition, ensure constant stirring and cooling to control the reaction temperature.
- Reflux:
- After the entire nitrating mixture has been added, place the R.B. flask in a water bath.
- Reflux the mixture at 60°C for about one and a half hours or until a yellow oily layer appears on the surface.
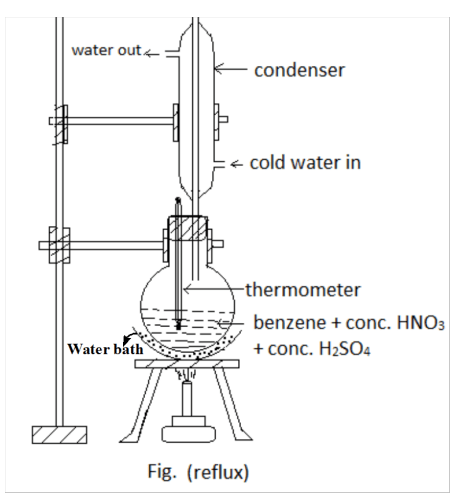
- Separation:
- Once the reaction is complete, transfer the contents of the R.B. flask to a separating funnel.
- Allow the layers to separate and then carefully separate the upper yellow oily layer (nitrobenzene).
- Washing:
- Wash the separated nitrobenzene layer with a sodium carbonate solution to remove any acidic impurities.
- Continue washing until the washings are neutral.
- Distillation:
- Transfer the washed nitrobenzene to a distillation apparatus.
- Distill the nitrobenzene at its boiling point of 210°C to purify it.

Properties of Nitrobenzene
Physical Properties:
- Appearance and Odor:
- Nitrobenzene is a pale yellow oily liquid with a characteristic bitter almond smell.
- Solubility:
- It is insoluble in water but is soluble in organic solvents.
- Polarity and Boiling Point:
- Nitrobenzene is a highly polar compound.
- It has a boiling point of 210°C.
Chemical Properties:
Reduction Reactions: Nitrobenzene undergoes various reduction reactions depending on the medium and reducing agents used.
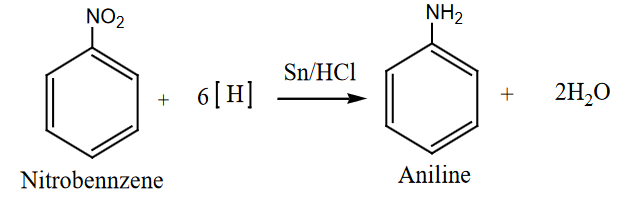
- Reduction in Acidic Medium:
- Nitrobenzene is reduced to aniline in the presence of a metal catalyst like Sn/HCl.

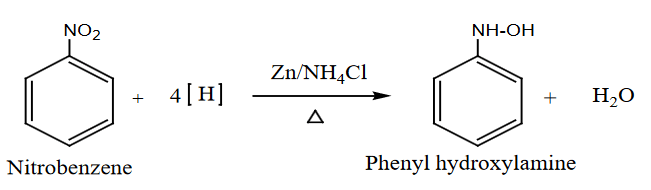
- Reduction in Neutral Medium:
- Nitrobenzene is reduced to phenylhydroxylamine in a neutral medium.
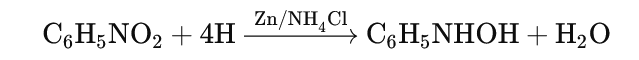
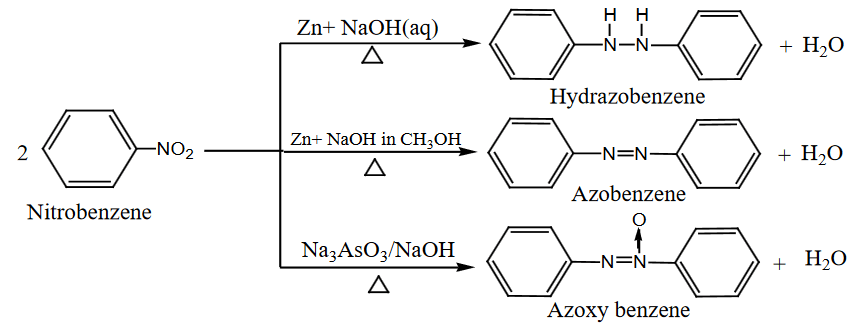
- Reduction in Alkaline Medium:
- Nitrobenzene can be reduced to various products depending on the reducing agent and the alkaline medium used.
- With Zn/NaOH (aqueous): Produces azobenzene.
- With Zn/NaOH (in methanol): Produces hydrazobenzene.
- With Na₃AsO₃/NaOH: Produces azoxybenzene.
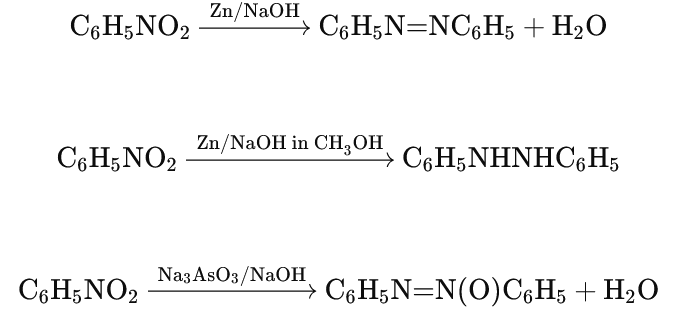
- Catalytic Reduction:
- Nitrobenzene is reduced to aniline in the presence of hydrogen (H₂) and a nickel (Ni), platinum (Pt), or palladium (Pd) catalyst.
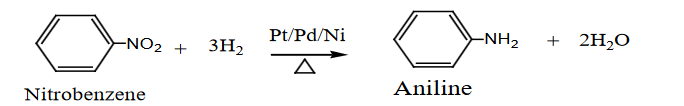
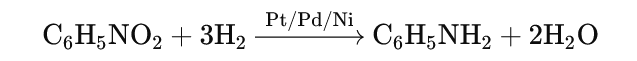
- Reduction with LiAlH₄:
- Nitrobenzene is reduced to azobenzene in the presence of lithium aluminum hydride (LiAlH₄).
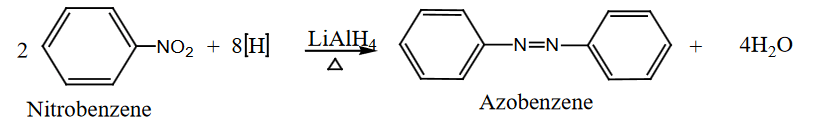
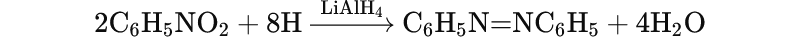
Electrophilic Substitution Reactions:
Due to the electron-withdrawing nature of the nitro group (-NO₂), nitrobenzene is a ring deactivator and directs incoming electrophiles to the meta position during electrophilic substitution reactions.
Halogenation:
- Reaction with chlorine (Cl₂) in the presence of FeCl₃ produces m-chloronitrobenzene.
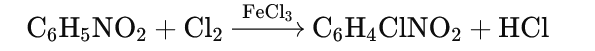
Sulphonation:
- Reaction with concentrated H₂SO₄ produces m-nitrobenzenesulfonic acid.
- C6H5NO2+H2SO4→C6H4(SO3H)NO2+H2O
Nitration:
- Reaction with fuming nitric acid (HNO₃) and fuming sulfuric acid (H₂SO₄) produces m-dinitrobenzene or m-trinitrobenzene depending on the conditions.
C6H5NO2+H2SO4→C6H4(SO3H)NO2+H2O
C6H3(NO2)2+HNO3+H2SO4→C6H2(NO2)3+H2O
Bromination:
- Reaction with bromine (Br₂) produces m-bromonitrobenzene.
C6H5NO2+Br2→C6H4BrNO2+HBr
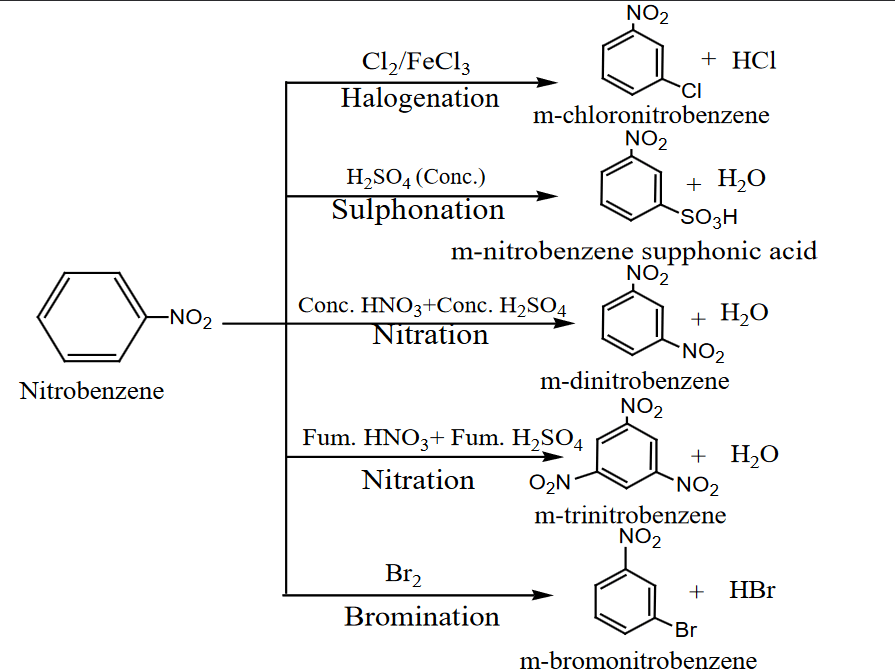
Uses of Nitrobenzene
Nitrobenzene finds several applications across different industries due to its versatile properties:
- Manufacture of Aniline:
- Nitrobenzene is a precursor in the production of aniline, a key chemical used in various industries including pharmaceuticals, rubber processing, and dye manufacturing.
- Shoe and Floor Polishes:
- It is used in the formulation of shoe polishes and floor polishes due to its solvent properties and ability to enhance gloss.
- High Boiling Inert Solvent:
- Nitrobenzene serves as a high boiling point solvent in industrial processes where high temperatures are involved or where other solvents might not be suitable.
- Dyes, Drugs, and Explosives:
- It is crucial in the manufacturing processes of dyes, pharmaceutical drugs, and explosives such as Trinitrotoluene (TNT) and Trinitrobenzene (TNB).
- Preparation of Organic Compounds:
- Nitrobenzene is used as a starting material in the synthesis of various important organic compounds, including intermediates for agrochemicals and specialty chemicals.

![NEB Class 12 Exam Routine 2081/2082 [2025]](https://iswori.com.np/wp-content/uploads/2025/02/neb-class-12-routine.png)
1 thought on “Nitro Compounds – NEB Class 12 Notes – Organic Chemistry”