Unit 10: Alcohols – Class 12 Chemistry
Class 12 Chemistry Chapter 10 Alcohols NEB Notes 2080. Class 12 Chemistry Unit 10 Alcohols Complete note, Exercise, Important Questions.
group, alcohol is formed. Alcohol can generally be represented by (R-OH) where R- is any alkyl group

Nomenclature
| Common Name | IUPAC Name | Molecular Formula |
|---|---|---|
| Methyl alcohol | Methanol | CH3OH |
| Ethyl alcohol | Ethanol | C2H5OH |
| Propyl alcohol | Propanol | C3H7OH |
| Butyl alcohol | Butanol | C4H9OH |
| Pentyl alcohol | Pentanol | C5H11OH |
| Hexyl alcohol | Hexanol | C6H13OH |
| Heptyl alcohol | Heptanol | C7H15OH |
| Octyl alcohol | Octanol | C8H17OH |
Classification of Alcohols (Monohydric alcohol)
Primary alcohols (1° Alcohols)
- If the -OH group is attached to a primary carbon (carbon attached to only one other carbon), the alcohol is called a primary alcohol.
- Example: Methanol (CH3OH) is a primary alcohol because the -OH group is attached to the primary carbon of methane (CH4).
Secondary alcohols (2° Alcohols)
- If the -OH group is attached to a secondary carbon (carbon attached to two other carbons), the alcohol is called a secondary alcohol.
- Example: Isopropanol (CH3-CH(OH)-CH3) is a secondary alcohol because the -OH group is attached to the secondary carbon of propane (CH3-CH2-CH3).
Tertiary alcohols (3° Alcohols)
- If the -OH group is attached to a tertiary carbon (carbon attached to three other carbons), the alcohol is called a tertiary alcohol.
- Example: Tert-butyl alcohol [(CH3)3-C-OH] is a tertiary alcohol because the -OH group is attached to the tertiary carbon of tert-butane [(CH3)3-C-H].
.jpeg)

Compounds having the same molecular formula but different
structural formula and chemical properties are called isomers
(Structural isomers) and the phenomenon is known as
isomerism. Alcohols exhibit only following three types of
structural isomerism.
1. Chain isomerism
Alcohols having same molecular formula but differ only in the
length of the carbon chain and properties are called chain
isomers and phenomenon is known as chain isomerism.
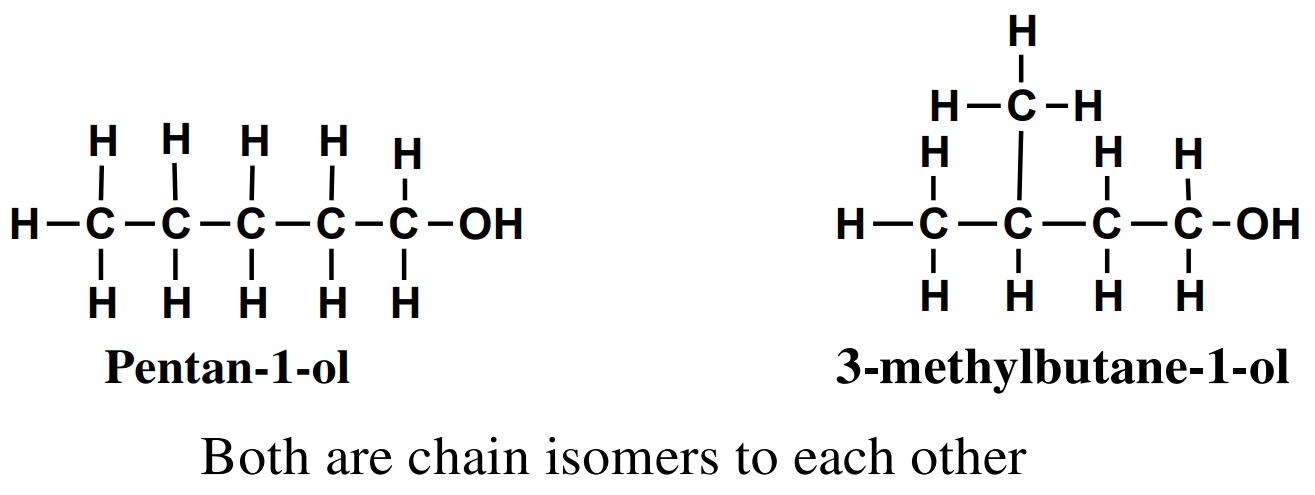
2. Positional isomerism
Alcohol having the same molecular formula, same carbon chain
length but differ only in the position of the –OH group in the
carbon chain are called positional isomers and phenomenon is
known as positional isomerism.
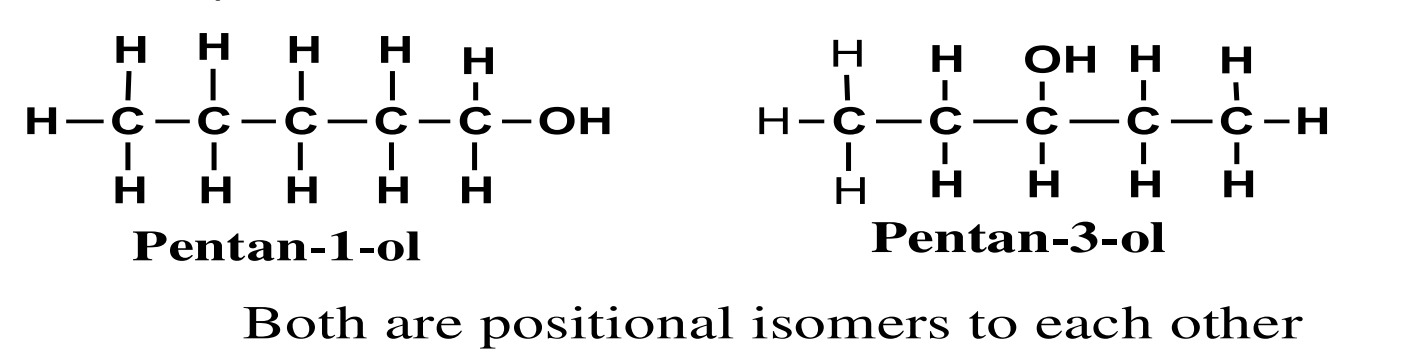
3. Functional isomerism
Alcohols are isomeric with ether. So alcohols can be functional
isomers to each other.

NaOH or KOH, alcohols are prepared.

alcohols. In case of unsymmetrical alkenes, the addition reaction
takes place in accordance with Markovnikov’s rule.

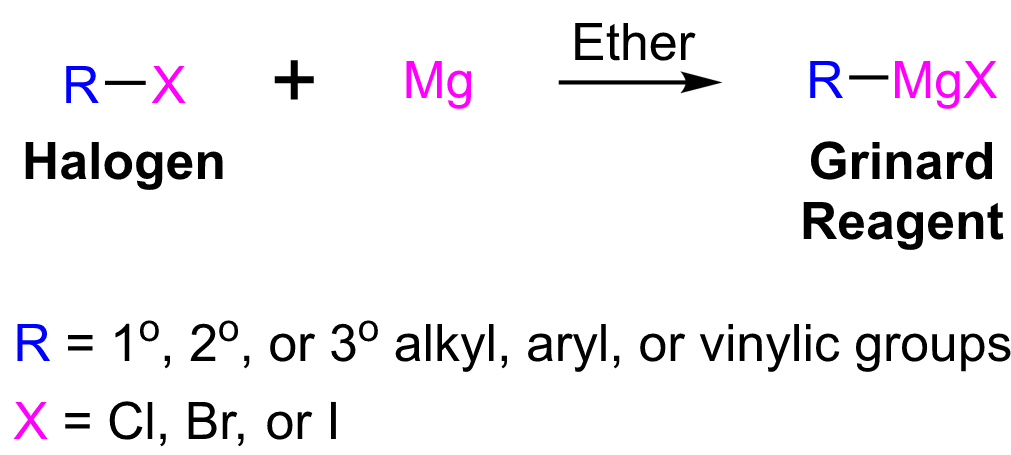 |
| Grignard Reagents Preparation |

reagent, always give primary (1° )alcohols
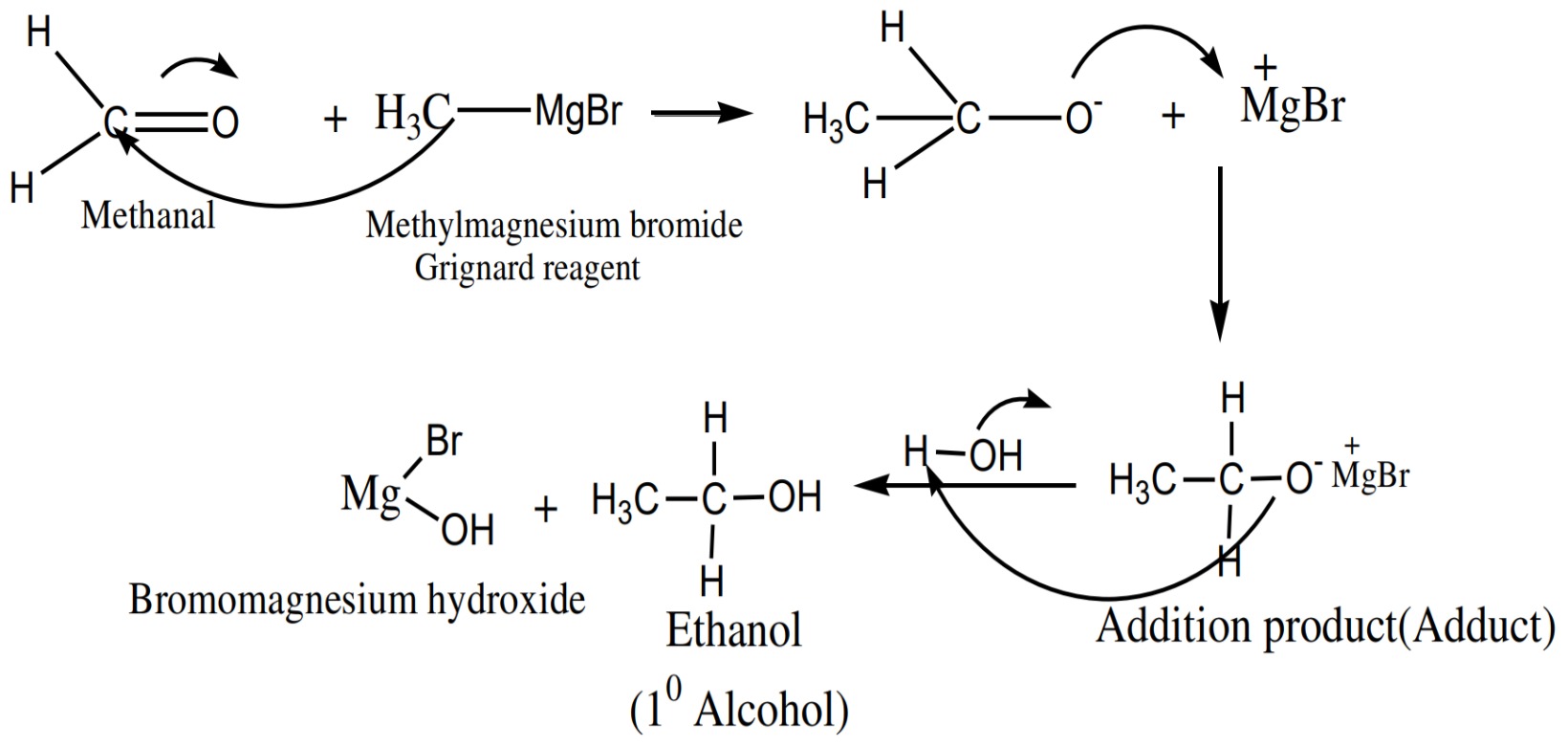alcohols.jpeg)
any Grignard reagent, always give secondary (2° ) alcohols.
%20alcohols%20with%20reaction%20mechanism.jpeg)
give tertiary (3° ) alcohols.
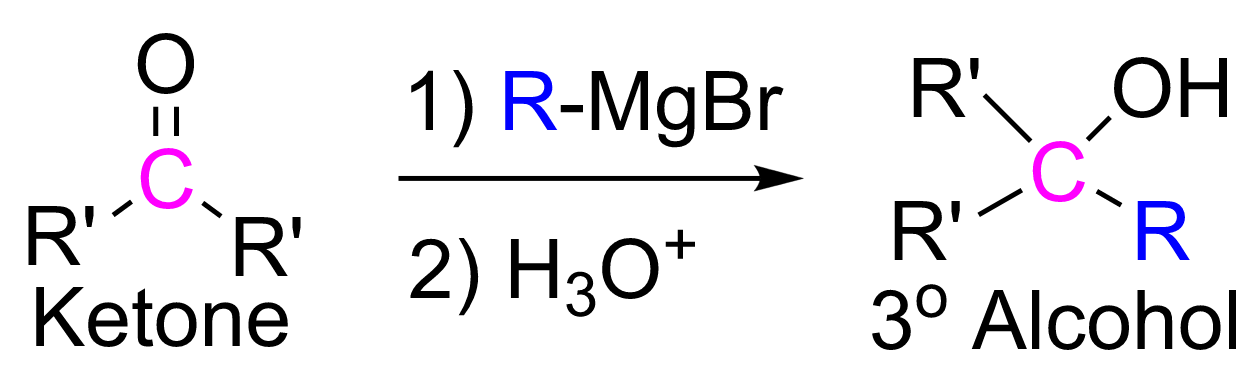%20alcohols..png)
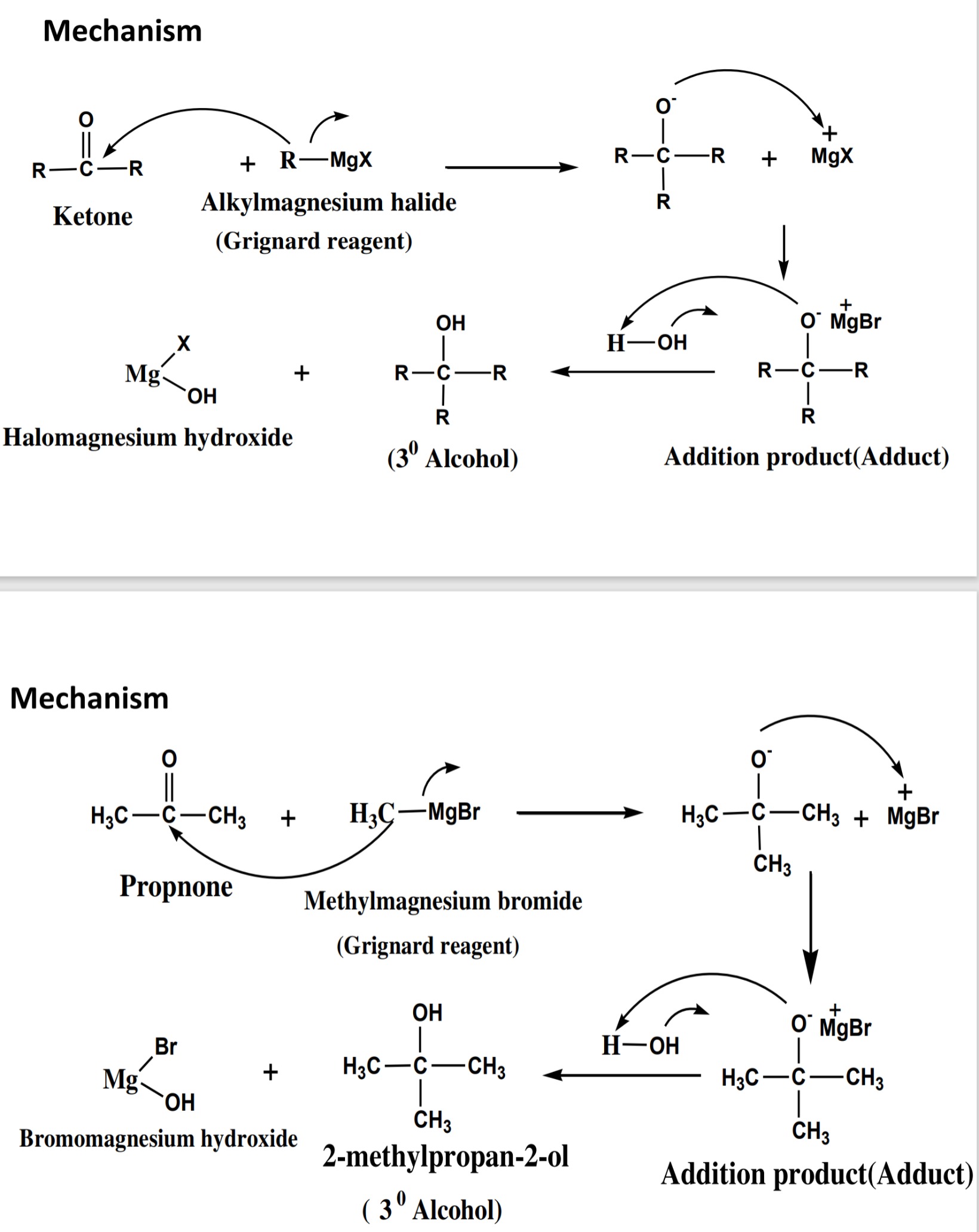%20alcohols%20mechanism.jpeg)
4. From primary amines
When primary amines are treated with nitrous acid. Alcohols are
formed. Nitrous acid is formed by the reaction of NaNO2
and
HCl. In this reaction nitrogen gas is also liberated.

presence of aq. Acidic or basic medium.
Fermentation is the slow decomposition of complex or higher
organic compound into simpler compounds by the action of
enzymes. The carbohydrates used for the fermentation are
sucrose, glucose ,fructose, molasses and sugar containing fruits
and starchy materials like wheat, rice, maize, barley, potato etc.
Fermentation is the old traditional method for the commercial
manufacture of ethyl alcohol.
Enzyme used is the unicellular plant material which contains
enzymes like invertase, diastase, maltase, zymase etc.
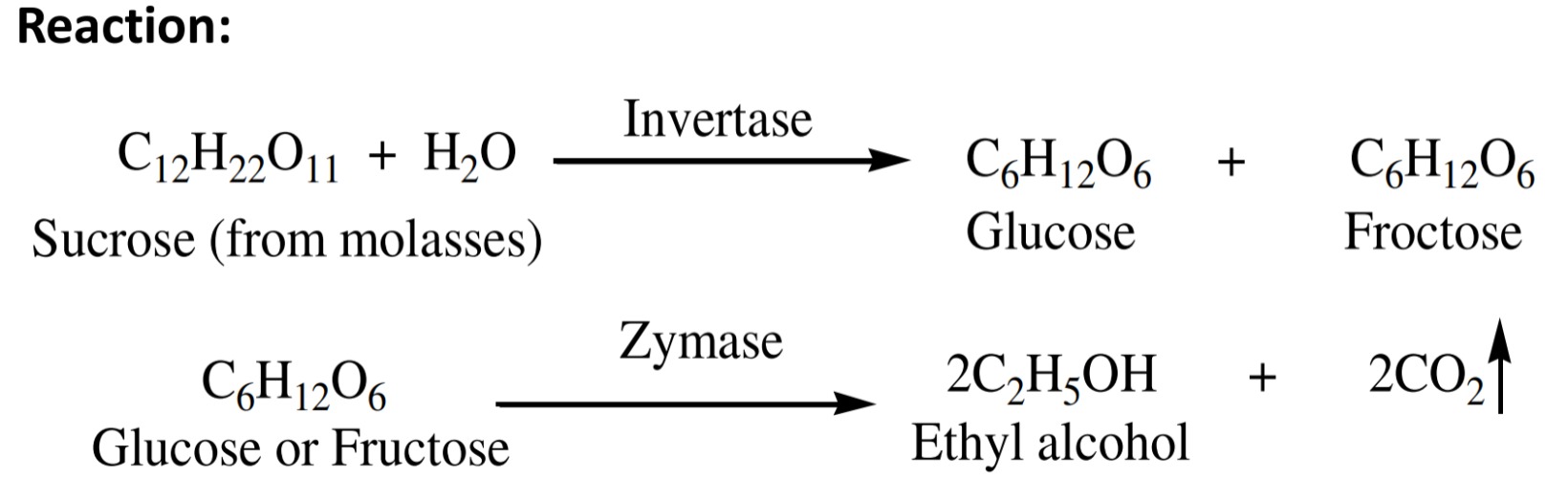
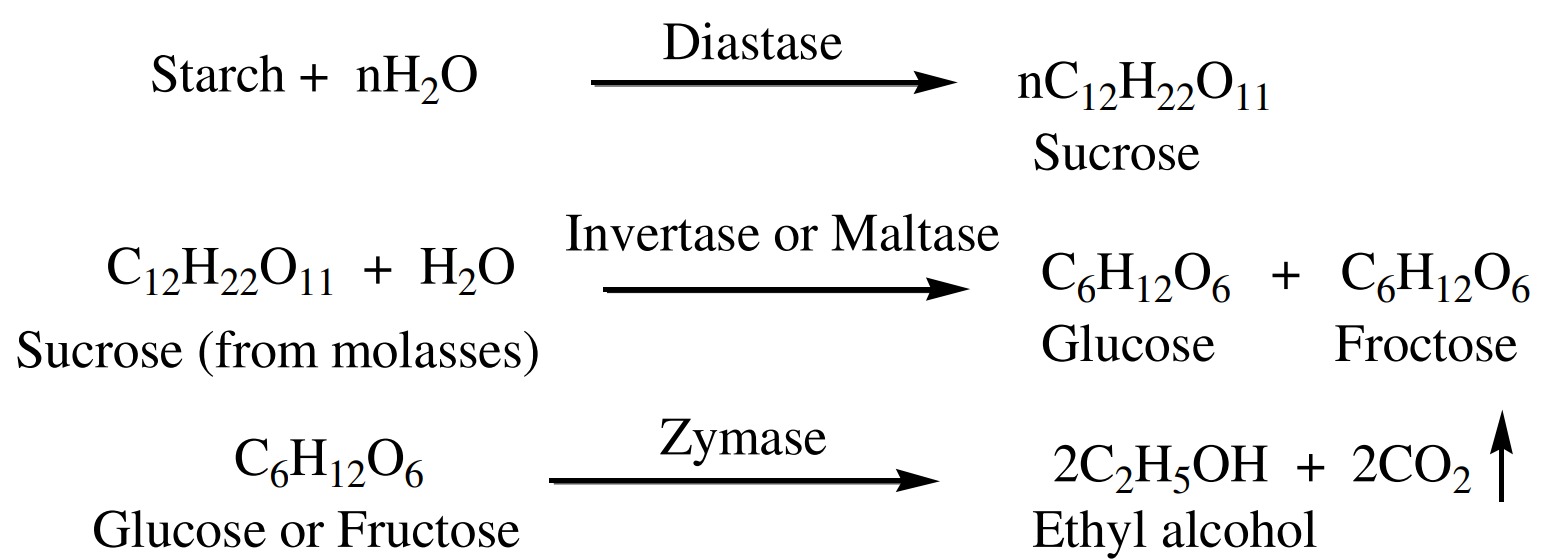
i. From fermentation of sugar
Molasses is the big source of sucrose, glucose, fructose. Etc.
Molasses is the dark brown colored mother liquor obtained
after the crystallization of cane sugar in the sugar industries.
Sucrose obtained from molasses when hydrolyzed in the
presence of enzyme ‘invertase’ give glucose or fructose.
or fructose then comes in contact with enzyme ‘zymase’ convert
into ethyl alcohol along with the evolution of CO2
gas.
The fermented liquor from the above process is called ‘wash’.
This wash contains 12-15% ethyl alcohol which can be obtained
in pure form by distillation process.
Starchy raw material used for the fermentation process are
rice, wheat, maize, barley, potato etc.
The raw materials are first thoroughly cocked or boiled with
water to release starch which is called ‘Mesh’.
The mesh is then
mixed with yeast (Enzyme) and kept for about 7-10 days or
more.
This fermented liquor is called ‘wash’ this wash contains 12-15
% ethyl alcohol is obtained impure form by distillation.
Favorable condition for fermentation:
- Yeast, a type of single-celled fungus, provides the enzymes needed
for fermentation.
- Little amount of ammonium sulphate or ammonium phosphate is
added as nutrient of yeast.
- If the yeast cells become too cold, fermentation happens very
slowly, or may not happen at all.
- If the yeast cells become too hot, their enzymes
become denatured and fermentation stops.
- sugars dissolved in water, and mixed with yeast
- an air lock to allow carbon dioxide out, while stopping air getting in warm temperature, 25-35°C
- The yeast dies when the ethanol concentration reaches about 15 %
- If air is present, the oxygen causes the ethanol to oxidize to
ethanoic acid, so the drink tastes of vinegar.
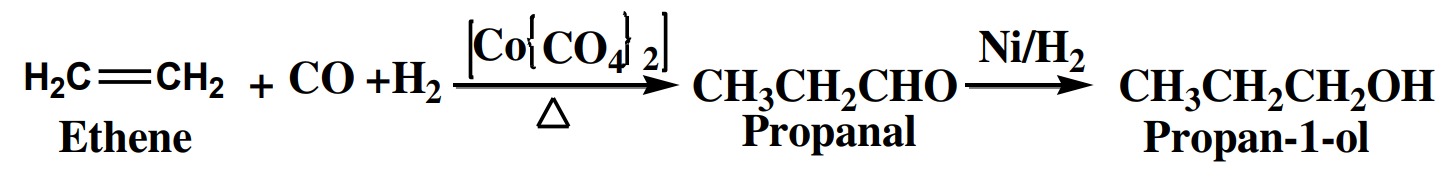
2. Oxo process
This is also the industrial process for manufacturing of alcohol above ethanol.
In this process alkenes are treated with (CO+H2
)the presence of cobalt carbonyl
catalyst (octacarbonyl dicobalt) to get aldehyde. This aldehyde on reduction in
the presence of Ni/H2 or Pt/H2
catalyst Catalytic hydrogenation) gives alcohol.
3. Hydroboration-oxidation of ethene
Alkenes react with diborane (B2H6
)
or (BH3
)2
undergo hydroboration to give
alkyl borane which on oxidation in the presence of H2O2
gives alcohol.

Properties of alcohols (Monohydric alcohols)
1. Physical properties
State:
Lower alcohols are colorless liquid with characteristic smell and burning
taste while higher alcohols are colorless waxy solids.
Solubility:
Lower alcohols are soluble in water due to presence of intermolecular
hydrogen bonding.
Solubility decreases with increase in the carbon chain or molecular
masses. This is due to the difference in the sizes of the alcohol and water
molecules.

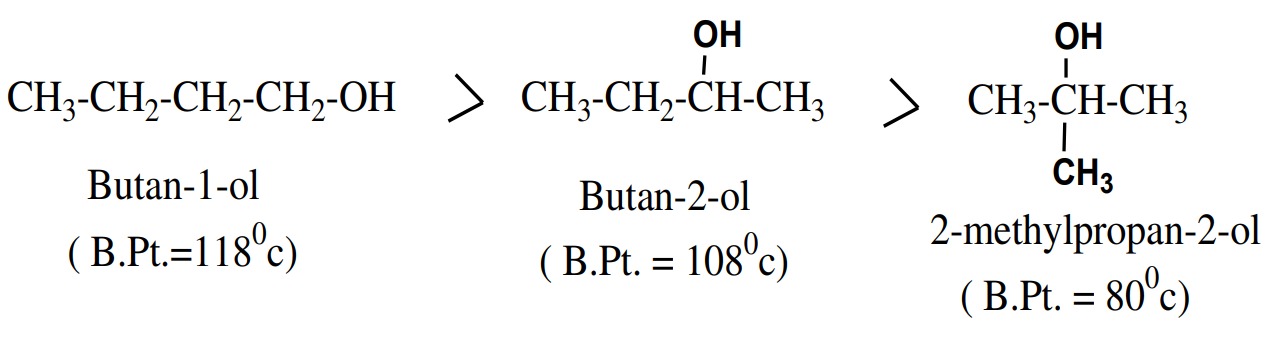
Boiling Point:
The boiling points of the alcohols are much higher than those of other
hydrocarbons having comparable molecular weight. It is because of
intermolecular hydrogen bonding formation.

- Boiling points of alcohols decreases with increase of branching.
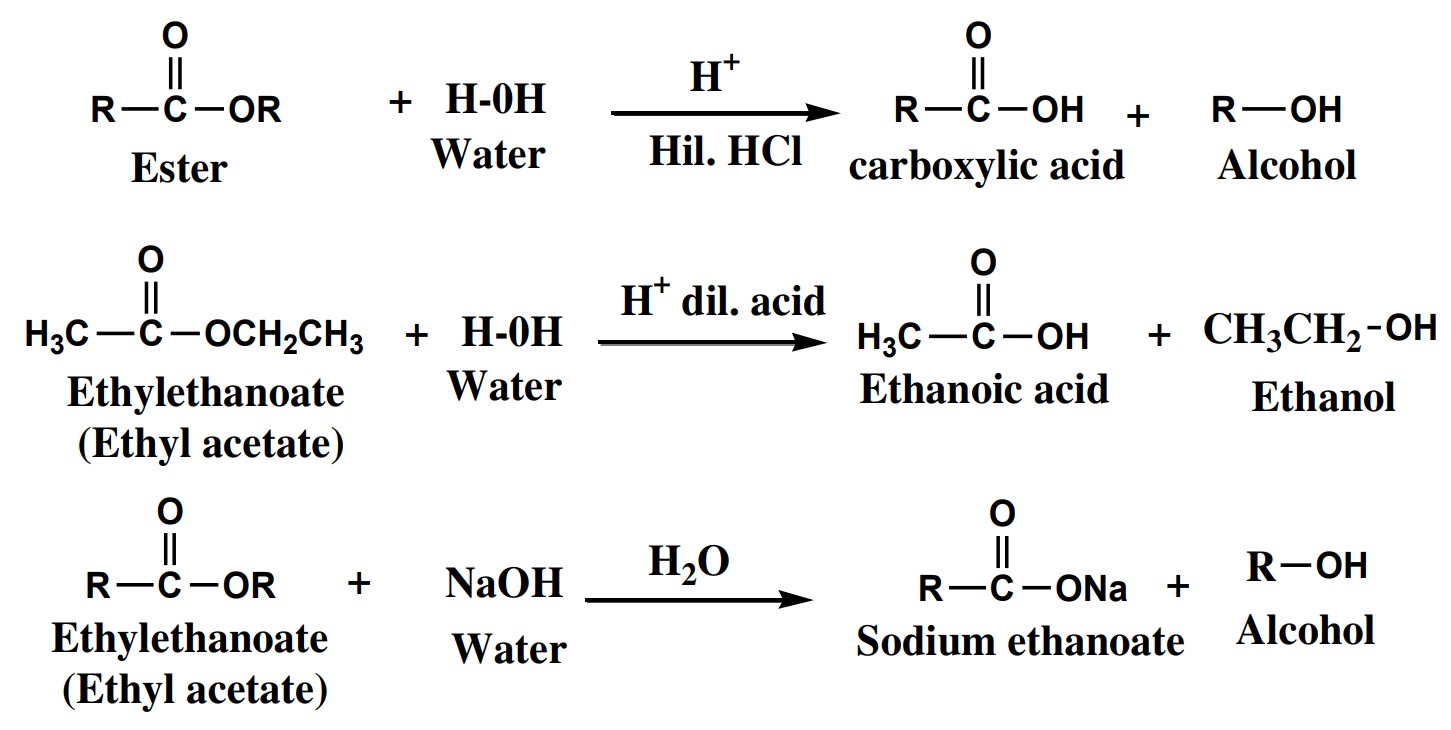
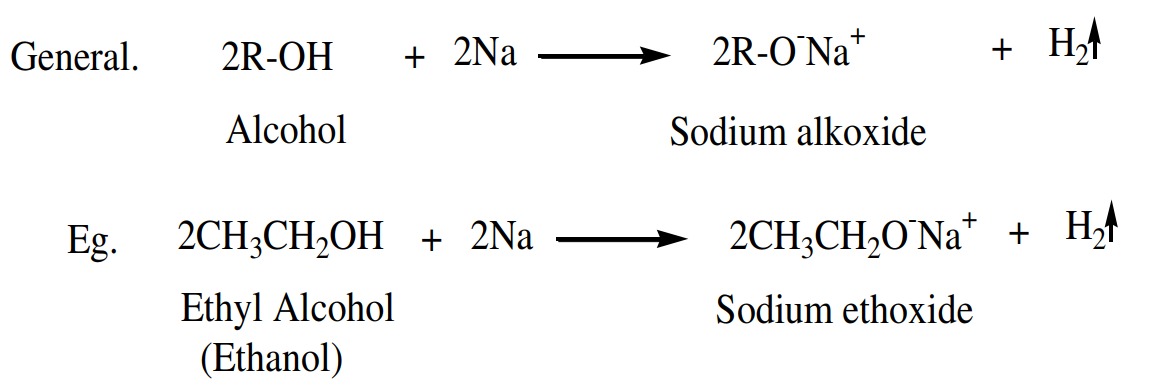
the presence of ether, breaking of the bond between -O-H takes place and metal
alkoxide and H2 gas released showing the acidic nature of alcohols.
.jpeg)
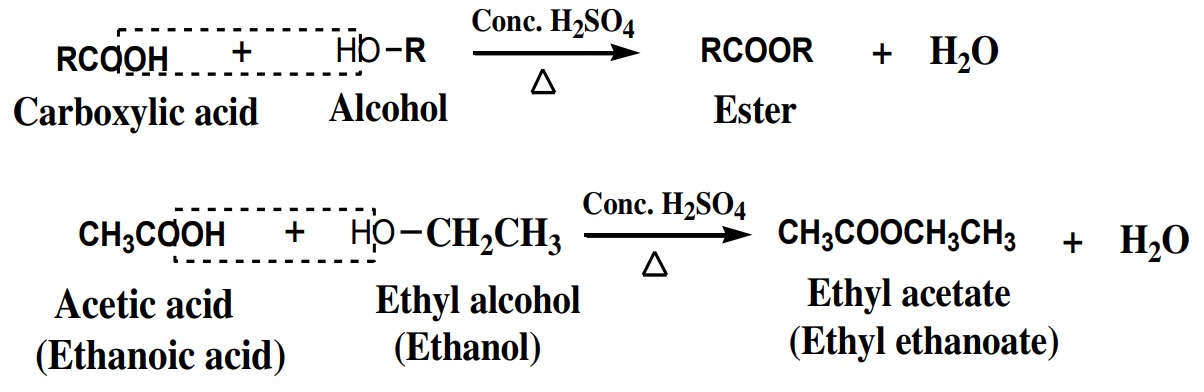
drops of conc. H2SO4
to give esters. This reaction is called
esterification reaction. Conc. H2SO4
acts as dehydrating agent.
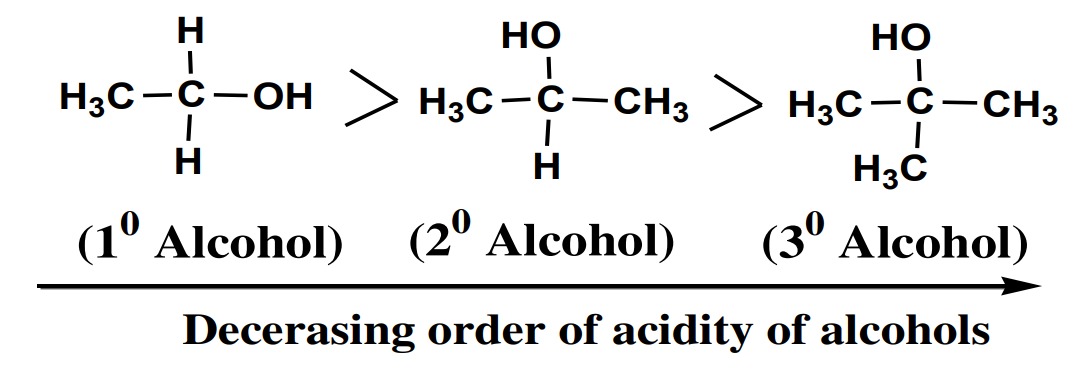
The order of basic strength of alcohols is given as:
Primary alcohol 〈 Secondary alcohol〈 Tertiary alcohols
,PX5
)
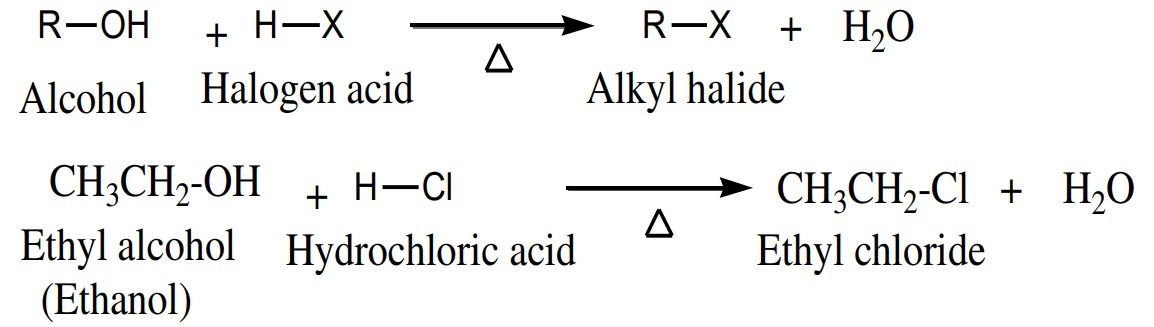
Alcohols are easily converted to alkyl halide when react with
phosphorus halides.
.jpeg)
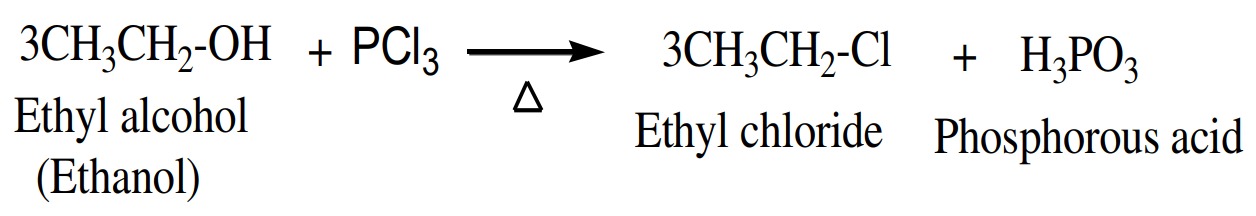
(5) Reaction with PX3
Alcohols also react with phosphorus trihalide to give alkyl
halide.
)
Alcohols react with thionyl chloride to give alkyl halides.
.jpeg)
(7) Reaction with H2SO4:
Ethyl alcohol reacts with conc. H2SO4
to give different products
at different temperatures.
(i) At 100℃:
Ethyl alcohol reacts with conc. H2SO4 at 100℃ to give ethyl
hydrogen sulphate.

(ii) At 140℃:
Ethyl alcohol reacts with conc. H2SO4 at 140℃ to give ethyl
diethyl ether (Ethoxy ethane).
..jpeg)
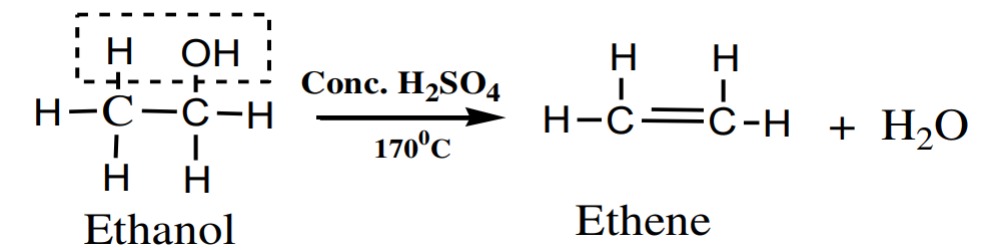
(iii) At 170℃:
Ethyl alcohol reacts with conc. H2SO4 at 170℃ to give ethene.
(8) Oxidation of 1° ,2° and 3° alcohols by oxidizing agents:
(i) 1° Alcohol:
Alcohols can easily be oxidized into aldehydes and ketones in
the presence of any oxidizing agents like acidic or alkaline
K2Cr2O7
, KMNO4
etc.

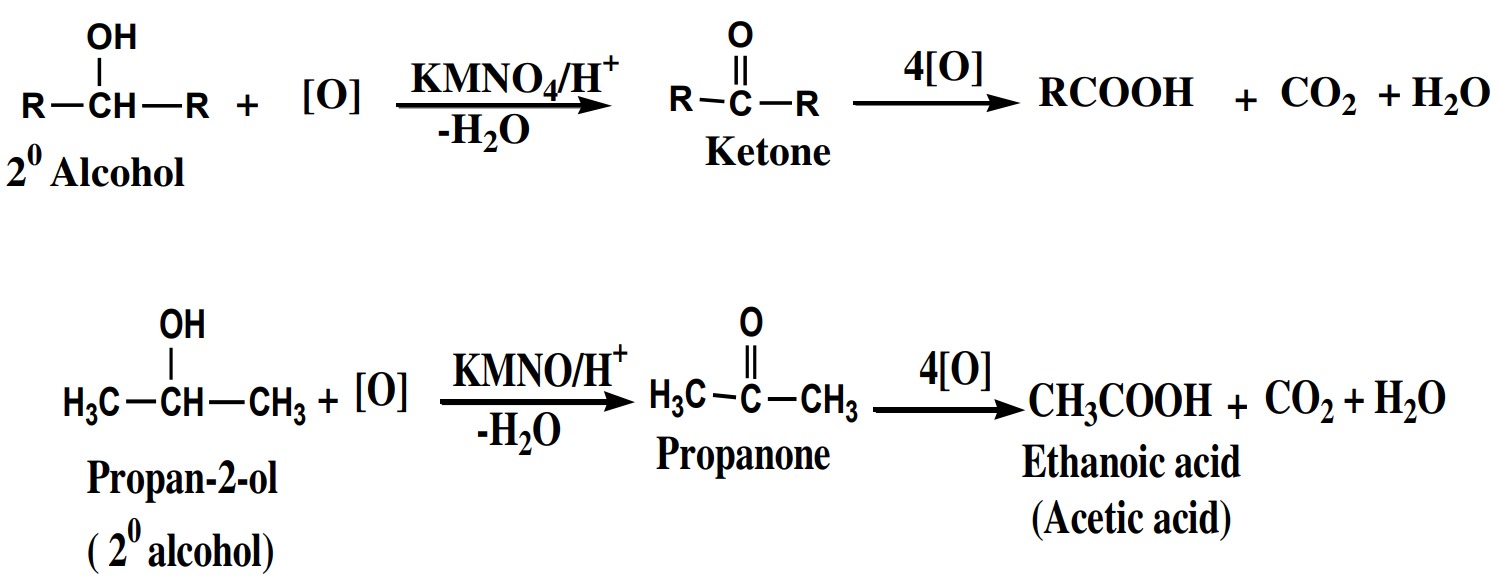
(ii) 2° Alcohol:
2° alcohols are oxidized into ketone with the same no of
carbon atoms. The ketones are further oxidized into carboxylic
acid with one carbon less than original ketones.
(iii) 3° Alcohol:
3° alcohols are not oxidized in ordinary condition because in 3° alcohols carbon containing –OH has no hydrogen atom.
(9) Reduction of alcohols (Catalytic dehydrogenation &
dehydration)
When alcohol vapors are passed through the red hot copper tube at
3000
C, Different class of alcohols give different products.
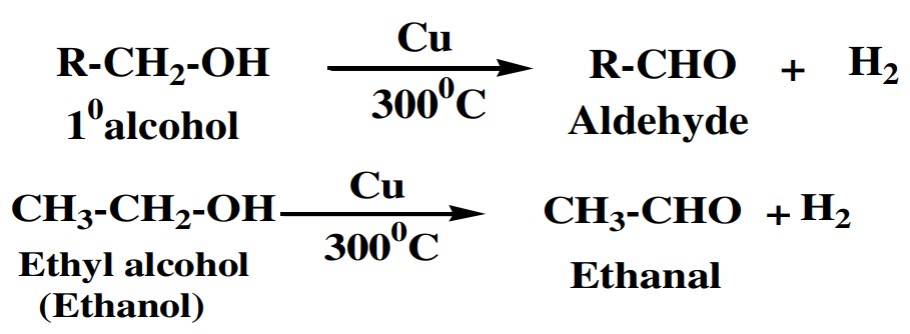
(i) 1° Alcohol:
1° or primary alcohols are dehydrogenated into
aldehydes.
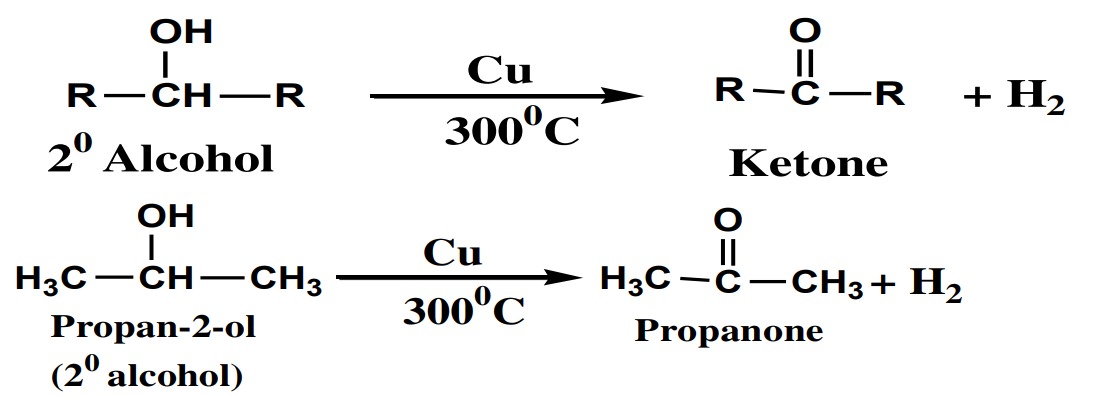
(ii) 2° Alcohol:
2° or secondary alcohols are dehydrogenated into
ketones.
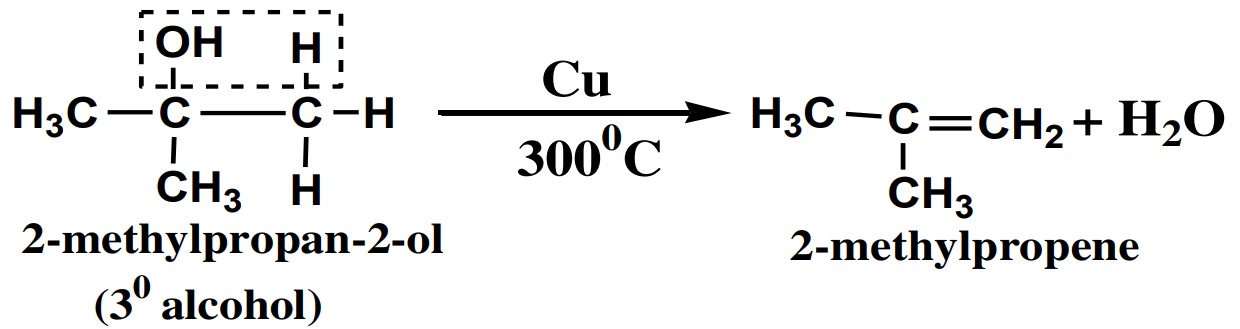
(iii) 3° Alcohol:
3° or tertiary alcohols are dehydrated into alkenes in the presence of Cu
catalyst at 3000C.
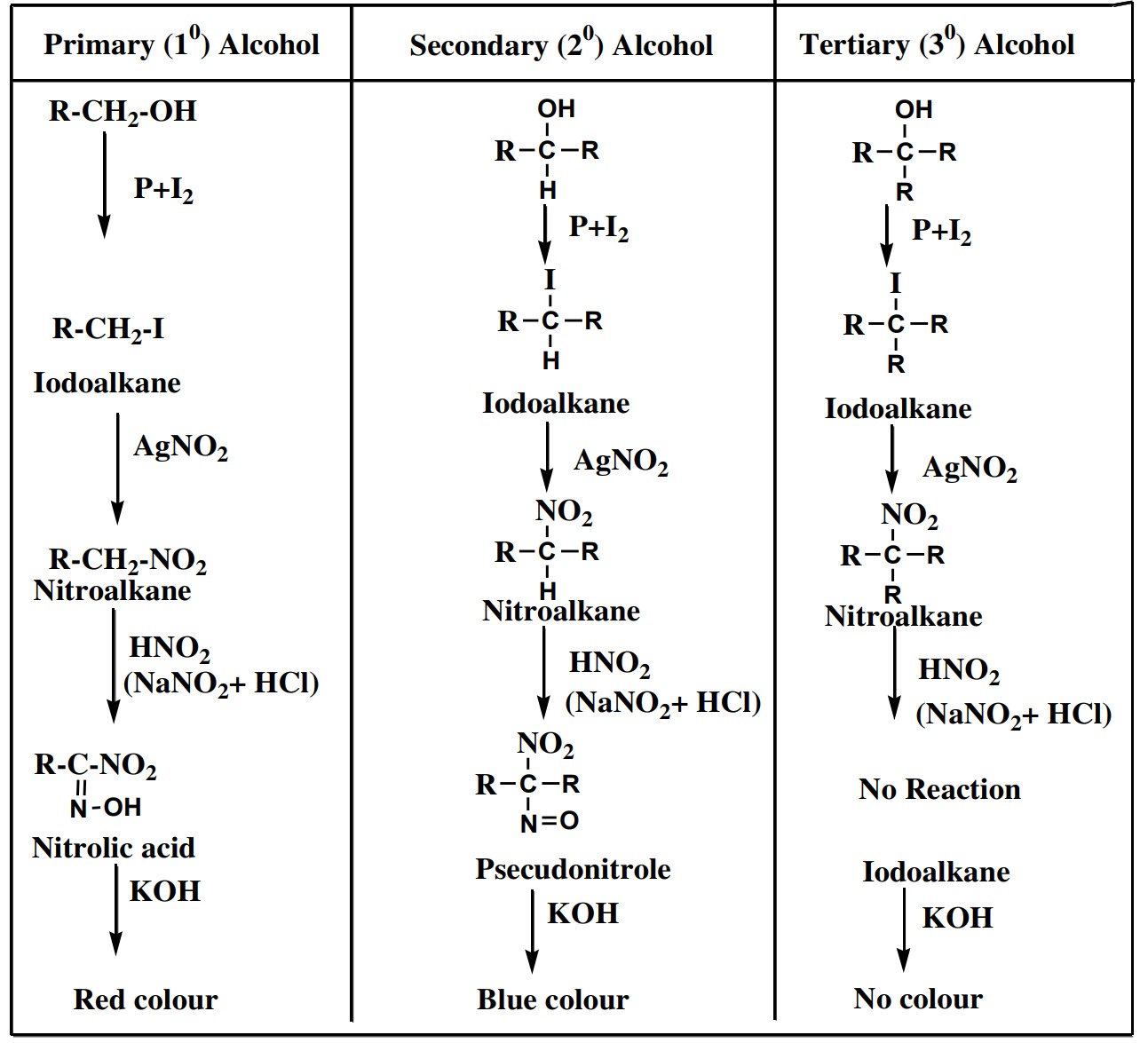
Distinction between 1°, 2° and 3° alcohols by Victor Meyer’s Method
There are several methods for distinguishing 1°, 2° and 3° alcohols but
most important method is Victor Meyer’s Method.
- (i) Oxidation method
- (ii) Catalytic dehydrogenation
- (iii) Victor Meyer’s Method
- (iv) Lucas Test
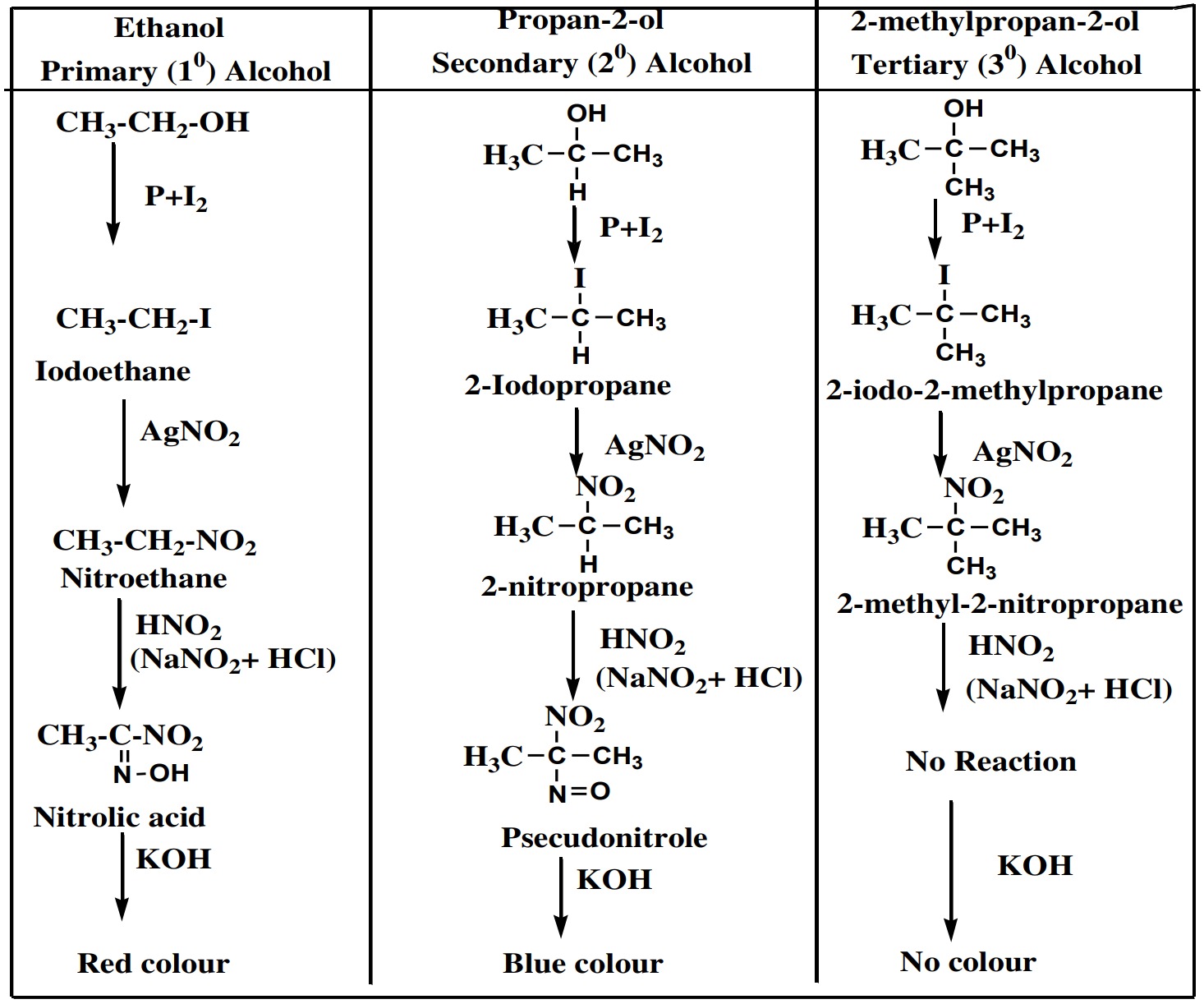
Victor Meyer’s Method is most important and widely used
method for distinguishing 1°, 2° and 3° alcohols. In this method
the given alcohol is first treated with phosphorus and iodine
solution (P+I2
) to give iodo-alkane which is then treated with
silver nitrite(AgNO2
) to give nitro alkane. The nitro alkane is
then treated with nitrous acid (HNO2
) and the resulting solution
is finally made alkaline by NaOH or KOH.
Following results are
obtained.
- (i) Primary alcohol gives red colour
- (ii) Secondary alcohol gives blue colour
- (iii) Tertiary alcohol gives no any colour
Table for distinguishing 1°, 2° and 3° alcohols is given as
Table for distinguishing 1°, 2° and 3° alcohols
| Test/Reagent | Primary (1°) Alcohol | Secondary (2°) Alcohol | Tertiary (3°) Alcohol |
|---|---|---|---|
| P+I2 | Iodoalkane | Iodoalkane | Iodoalkane |
| AgNO2 | Nitroalkane | Nitroalkane | Nitroalkane |
| HNO2 (NaNO2 + HCl) (Pseudonitrole Iodoalkane) | Nitrolic acid | Nitrolic acid | No Reaction |
| KOH | Red colour | Blue colour | No colour |
Victor Meyer’s Method – Table for distinguishing 1°, 2° and 3° alcohols
Victor Meyer’s Method Example – Table for distinguishing 1°, 2° and 3° alcohols
Lucas Test
In This test the unknown alcohol is treated with the Lucas reagent (HCl + ZnCl2
) .
This is the reaction of alcohol with HCl in the presence of dilute HCl.
The time taken for the reaction to occur is important to know the class of
alcohols.
The occurrence of reaction can be observed by the appearance of
white turbidity or cloudiness.
- (i) For 1° alcohol, reaction occurs only after heating.
- (ii) For 2° alcohol, reaction occurs within five minutes.
- (iii) For 3° alcohol, reaction occurs immediately.
Table:
| Class of Alcohol | Reaction with Lucas Reagent | Observation |
|---|---|---|
| Primary (1°) Alcohol | R-CH2-OH + ZnCl2 → R-CH2-Cl + HCl (Chloroalkane) | Reaction occurs only after heating |
| Secondary (2°) Alcohol | R2CH-OH + ZnCl2 + HCl → R2CH-Cl + HCl + HCl (Chloroalkane) | Reaction occurs within five minutes |
| Tertiary (3°) Alcohol | R3C-OH + ZnCl2 + 2 HCl → R3C-Cl + ZnCl2 + 2 H2O (Chloroalkane) | Reaction occurs immediately |
Lucas Test Table:

Test for ethyl alcohol:
- (i) Esterification Test (Already Studied)
- (ii) Iodoform test
more soon………….

![NEB Class 12 Exam Routine 2082 [2025] 3 NEB Class 12 Exam Routine 2081/2082 [2025]](https://iswori.com.np/wp-content/uploads/2025/02/neb-class-12-routine.png)
