Aromatic Aldehydes and Ketones
Class 12 Chemistry Unit 13 Aromatic Aldehydes and Ketones Complete Note, Exercise, Important Questions.

Aromatic Aldehydes and Ketones Syllabus
13.2 Aromatic Aldehydes and Ketones
13.2.1 Preparation of Benzaldehyde from Toluene and Acetophenone from Benzene
13.2.2 Properties of Benzaldehyde
13.2.2.1 Perkin Condensation
13.2.2.2 Benzoin Condensation
13.2.2.3 Cannizzaro’s Reaction
13.2.2.4 Electrophilic Substitution Reaction
more: NEB Class 12 Chemistry Syllabus
The compounds in which the carbonyl group is directly bonded to aromatic ring are called aromatic aldehydes and ketones. The compound in which the aldehyde functional group (-CHO) is directly bonded to aromatic ring are called aromatic aldehydes.

The compound in which the –CHO group is bonded to side chain of aromatic ring are called aryl substituted aliphatic aldehyde.
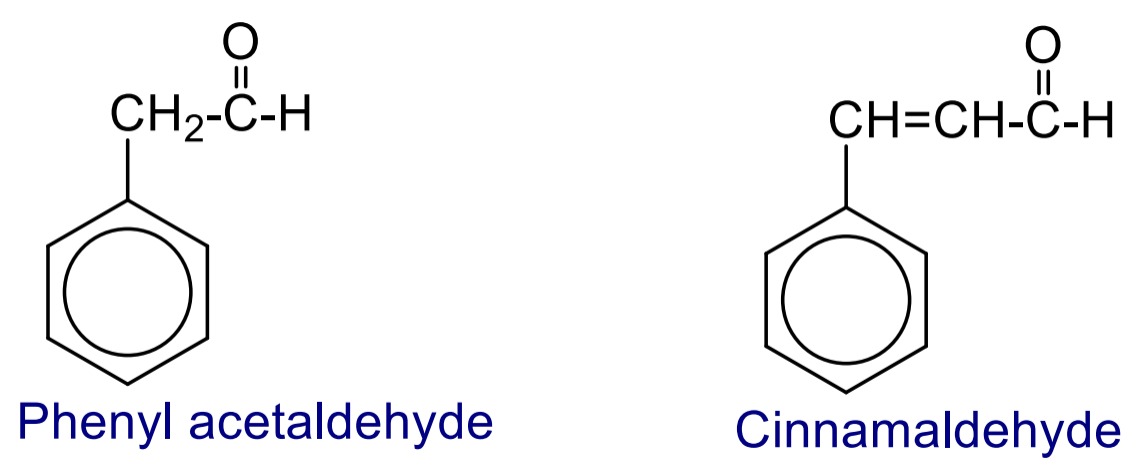
The compound in which the keto functional group (>C=O) group is directly bonded to aromatic ring are called aromatic ketone.
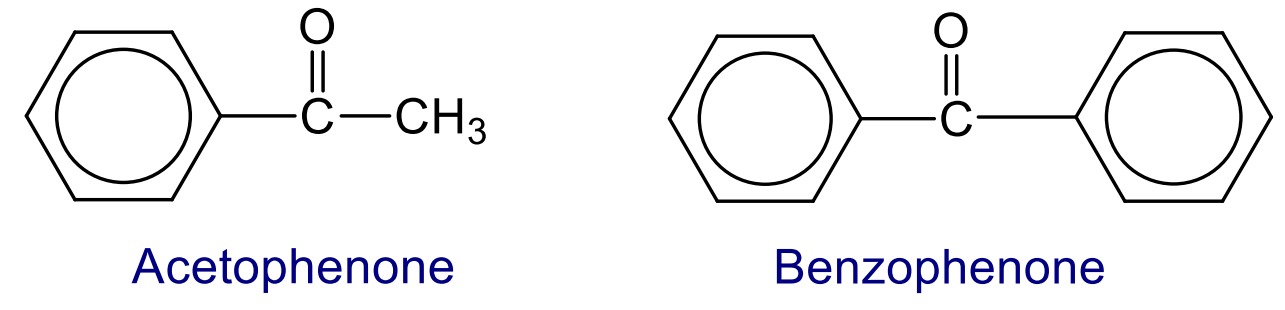
Nomenclature of aromatic aldehydes and ketones

Benzaldehyde
Benzaldehyde is an organic compound with the chemical formula C6H5CHO. It is an aromatic aldehyde, meaning it contains both an aromatic ring and an aldehyde functional group (-CHO) attached to it. The compound is characterized by its distinct almond-like odor, which is responsible for its use in flavoring and fragrance applications.
Preparation
Benzaldehyde can be prepared by various methods, including oxidation of benzyl alcohol using an oxidizing agent such as chromic acid, potassium permanganate, or sodium dichromate. It can also be obtained from toluene via a side-chain oxidation process.
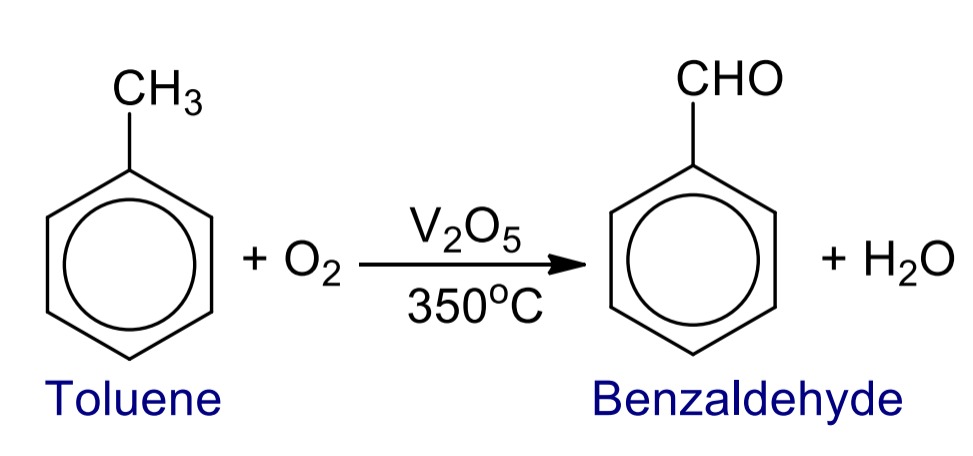
1. Oxidation of toluene
When toluene is oxidized with chromium trioxide in presence of acetic anhydride followed by alkaline hydrolysis or with air in presence of V₂O₅ catalyst at 350˚C then benzaldehyde is formed.
- Chromic Acid Oxidation: Toluene is oxidized with chromium trioxide (CrO3) in the presence of acetic anhydride. This reaction yields benzaldehyde along with chromium sulfate and water as byproducts. Subsequently, the benzaldehyde product can undergo alkaline hydrolysis with sodium hydroxide (NaOH) to form benzoic acid and sodium formate.

- Vapor Phase Oxidation: Alternatively, toluene can be oxidized with air as the oxidizing agent in the presence of a vanadium pentoxide (V₂O₅) catalyst at a temperature of 350˚C. This process leads to the formation of benzaldehyde as the main product.
Acetophenone
Acetophenone is an organic compound with the chemical formula C8H8O. It is a ketone, characterized by a carbonyl group (-C=O) attached to an aromatic ring. The compound has a sweet and pleasant aroma, which gives it its name as it resembles the smell of acetic acid (vinegar) and benzaldehyde combined.
Preparation
Acetophenone can be synthesized through various methods, including the Friedel-Crafts acylation of benzene using acetyl chloride or acetic anhydride as an acylating agent. This reaction is catalyzed by Lewis acids such as aluminum chloride (AlCl3) or iron(III) chloride (FeCl3).
From benzene (Friedel-Craft’s acylation)
When benzene is heated with acetyl chloride or acetic anhydride in presence of anhydrous AlCl₃ then acetophenone is formed.
.jpeg)
Chemical properties of benzaldehyde
[A] Reaction due to –CHO group different from aliphatic aldehyde
1. Perkin’s condensation reaction
When benzaldehyde is heated with acetic anhydride in presence of sodium acetate then α,β-unsaturated acid is formed.

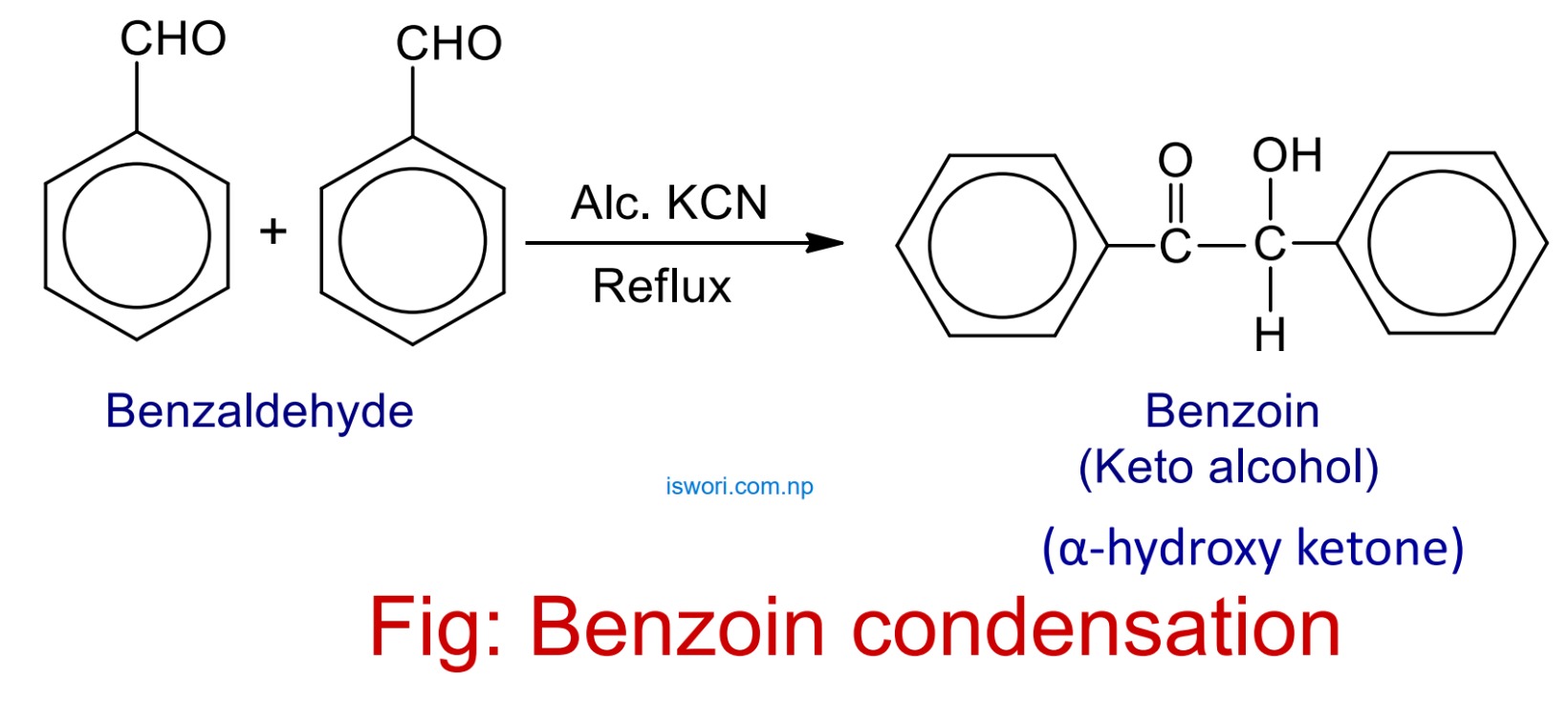
2. Benzoin condensation
When two molecule of benzaldehyde are refluxed with alcoholic KCN then self condensation reaction occurs to give α-hydroxy ketone (benzoin).
3. Cannizzaro’s reaction
When two molecule of benzaldehyde undergoes self reduction and oxidation in presence of Conc. NaOH then benzaldehyde and sodium benzoate are formed.

[B] Reaction due to benzene ring (Electrophilic substitution reaction)
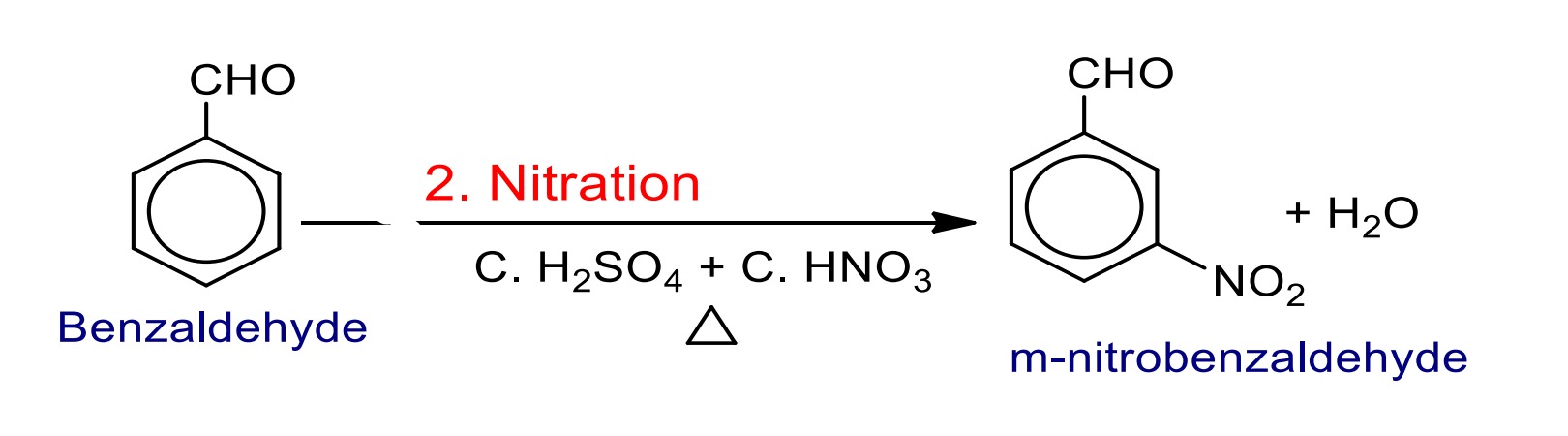
The –CHO group in benzaldehyde is ring deactivating group due to negative inductive effect and meta directing group because it directs an incoming electrophile at meta position (due to resonance effect) towards electrophilic substitution reaction.
.jpeg)
1. Halogenation

2. Nitration
3. Sulphonation


![NEB Class 12 Exam Routine 2081/2082 [2025]](https://iswori.com.np/wp-content/uploads/2025/02/neb-class-12-routine.png)
1 thought on “Aldehyde and Ketone – NEB Class 12 Chemistry Notes”